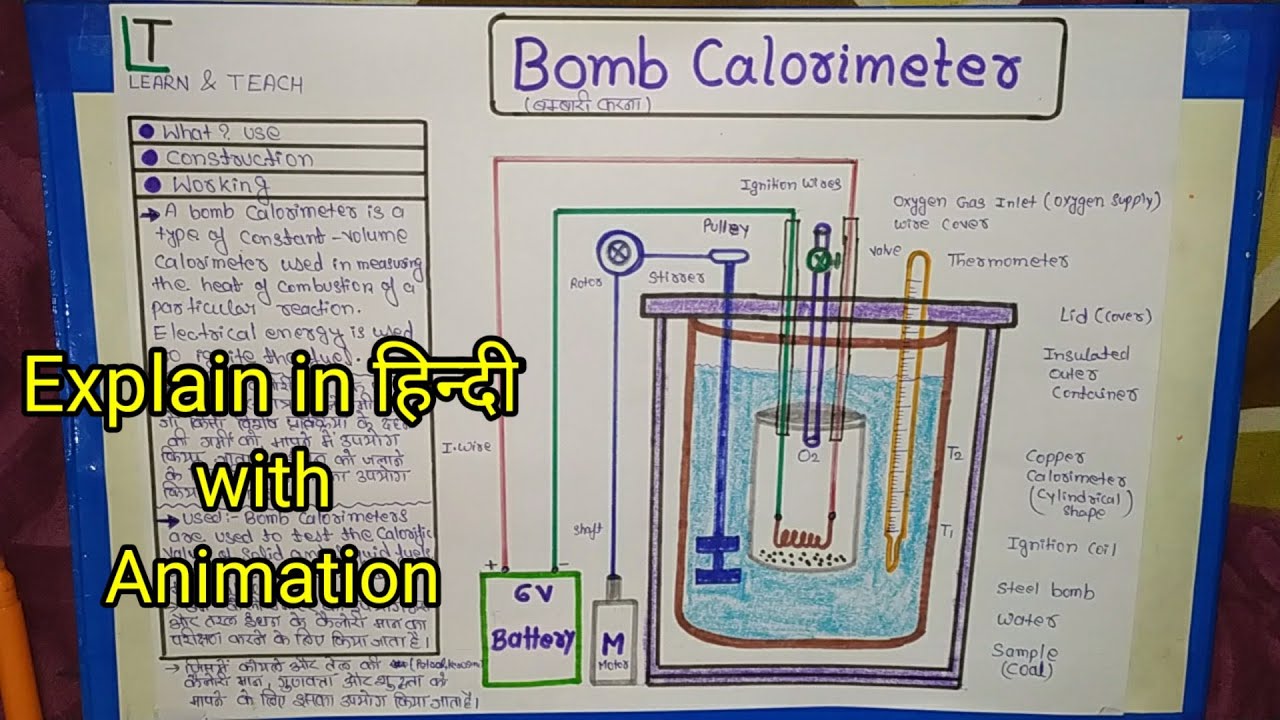
Explain The Construction Of Calorimeter Draw The Necessary Figure . The inner vessel, made of copper, is (practically). What is the difference between heat and. Figure shows the construction of a calorimeter. figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat. Explain the construction of a calorimeter. It mainly consists of a metallic vessel made of materials which are good conductors. Calculate and interpret heat and related properties using typical calorimetry data Like a thermo flask, a calorimeter has two vessels. explain the technique of calorimetry; click here👆to get an answer to your question ️ write brief answers : How does it differ from the thermometer used in laboratory? a calorimeter is a device used for heat measurements necessary for calorimetry. Like a thermo flask, a calorimeter has two vessels. Figure shows the construction of a calorimeter.
from www.animalia-life.club
a calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of materials which are good conductors. click here👆to get an answer to your question ️ write brief answers : The inner vessel, made of copper, is (practically). Like a thermo flask, a calorimeter has two vessels. Figure shows the construction of a calorimeter. How does it differ from the thermometer used in laboratory? Figure shows the construction of a calorimeter. Explain the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels.
Calorimeter Diagram
Explain The Construction Of Calorimeter Draw The Necessary Figure It mainly consists of a metallic vessel made of materials which are good conductors. Like a thermo flask, a calorimeter has two vessels. It mainly consists of a metallic vessel made of materials which are good conductors. Figure shows the construction of a calorimeter. Figure shows the construction of a calorimeter. click here👆to get an answer to your question ️ write brief answers : How does it differ from the thermometer used in laboratory? figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat. What is the difference between heat and. Calculate and interpret heat and related properties using typical calorimetry data Explain the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels. explain the technique of calorimetry; The inner vessel, made of copper, is (practically). a calorimeter is a device used for heat measurements necessary for calorimetry.
From courses.lumenlearning.com
Calorimetry General Chemistry Explain The Construction Of Calorimeter Draw The Necessary Figure figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat. What is the difference between heat and. Like a thermo flask, a calorimeter has two vessels. Figure shows the construction of a calorimeter. The inner vessel, made of copper, is (practically). It mainly consists of a. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From www.thoughtco.com
Calorimeter Definition in Chemistry Explain The Construction Of Calorimeter Draw The Necessary Figure a calorimeter is a device used for heat measurements necessary for calorimetry. click here👆to get an answer to your question ️ write brief answers : What is the difference between heat and. figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat. Like a. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From www.animalia-life.club
Calorimeter Diagram Explain The Construction Of Calorimeter Draw The Necessary Figure Figure shows the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels. Calculate and interpret heat and related properties using typical calorimetry data It mainly consists of a metallic vessel made of materials which are good conductors. The inner vessel, made of copper, is (practically). Like a thermo flask, a calorimeter has two vessels. explain. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Explain The Construction Of Calorimeter Draw The Necessary Figure Figure shows the construction of a calorimeter. Explain the construction of a calorimeter. It mainly consists of a metallic vessel made of materials which are good conductors. a calorimeter is a device used for heat measurements necessary for calorimetry. Like a thermo flask, a calorimeter has two vessels. figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From www.animalia-life.club
Calorimeter Diagram Explain The Construction Of Calorimeter Draw The Necessary Figure Figure shows the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels. How does it differ from the thermometer used in laboratory? click here👆to get an answer to your question ️ write brief answers : explain the technique of calorimetry; Explain the construction of a calorimeter. It mainly consists of a metallic vessel made. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Explain The Construction Of Calorimeter Draw The Necessary Figure click here👆to get an answer to your question ️ write brief answers : What is the difference between heat and. Figure shows the construction of a calorimeter. How does it differ from the thermometer used in laboratory? Figure shows the construction of a calorimeter. a calorimeter is a device used for heat measurements necessary for calorimetry. Like a. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Explain The Construction Of Calorimeter Draw The Necessary Figure Like a thermo flask, a calorimeter has two vessels. The inner vessel, made of copper, is (practically). Figure shows the construction of a calorimeter. Figure shows the construction of a calorimeter. Explain the construction of a calorimeter. explain the technique of calorimetry; figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From www.animalia-life.club
Calorimeter Diagram Explain The Construction Of Calorimeter Draw The Necessary Figure Like a thermo flask, a calorimeter has two vessels. click here👆to get an answer to your question ️ write brief answers : Explain the construction of a calorimeter. Calculate and interpret heat and related properties using typical calorimetry data How does it differ from the thermometer used in laboratory? a calorimeter is a device used for heat measurements. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Explain The Construction Of Calorimeter Draw The Necessary Figure The inner vessel, made of copper, is (practically). click here👆to get an answer to your question ️ write brief answers : Like a thermo flask, a calorimeter has two vessels. What is the difference between heat and. Calculate and interpret heat and related properties using typical calorimetry data How does it differ from the thermometer used in laboratory? . Explain The Construction Of Calorimeter Draw The Necessary Figure.
From askfilo.com
Explain the construction of a calorimeter. Draw the necessary figure. (0.. Explain The Construction Of Calorimeter Draw The Necessary Figure Like a thermo flask, a calorimeter has two vessels. Figure shows the construction of a calorimeter. a calorimeter is a device used for heat measurements necessary for calorimetry. Calculate and interpret heat and related properties using typical calorimetry data figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From kaffee.50webs.com
Lab Calorimetry Explain The Construction Of Calorimeter Draw The Necessary Figure How does it differ from the thermometer used in laboratory? It mainly consists of a metallic vessel made of materials which are good conductors. explain the technique of calorimetry; Explain the construction of a calorimeter. figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat.. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Explain The Construction Of Calorimeter Draw The Necessary Figure It mainly consists of a metallic vessel made of materials which are good conductors. figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat. Figure shows the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels. explain the technique of calorimetry;. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Explain The Construction Of Calorimeter Draw The Necessary Figure explain the technique of calorimetry; Like a thermo flask, a calorimeter has two vessels. Like a thermo flask, a calorimeter has two vessels. Calculate and interpret heat and related properties using typical calorimetry data The inner vessel, made of copper, is (practically). figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From www.slideserve.com
PPT ACTIVE LEARNING PROCESS PowerPoint Presentation ID2492560 Explain The Construction Of Calorimeter Draw The Necessary Figure It mainly consists of a metallic vessel made of materials which are good conductors. Like a thermo flask, a calorimeter has two vessels. figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat. How does it differ from the thermometer used in laboratory? Like a thermo. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From www.researchgate.net
Sketch of the threelayercalorimeter. Download Scientific Diagram Explain The Construction Of Calorimeter Draw The Necessary Figure It mainly consists of a metallic vessel made of materials which are good conductors. Calculate and interpret heat and related properties using typical calorimetry data Figure shows the construction of a calorimeter. How does it differ from the thermometer used in laboratory? Like a thermo flask, a calorimeter has two vessels. Like a thermo flask, a calorimeter has two vessels.. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From brainly.in
explain the construction of calorimeter. Draw the necessary figure Explain The Construction Of Calorimeter Draw The Necessary Figure What is the difference between heat and. Figure shows the construction of a calorimeter. The inner vessel, made of copper, is (practically). Figure shows the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels. explain the technique of calorimetry; Explain the construction of a calorimeter. click here👆to get an answer to your question ️. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From www.animalia-life.club
Calorimeter Diagram Explain The Construction Of Calorimeter Draw The Necessary Figure Figure shows the construction of a calorimeter. The inner vessel, made of copper, is (practically). Like a thermo flask, a calorimeter has two vessels. What is the difference between heat and. Explain the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels. a calorimeter is a device used for heat measurements necessary for calorimetry. Calculate. Explain The Construction Of Calorimeter Draw The Necessary Figure.
From celxxkrb.blob.core.windows.net
Proper Use Of Calorimeter at Tomas Tudor blog Explain The Construction Of Calorimeter Draw The Necessary Figure Figure shows the construction of a calorimeter. Like a thermo flask, a calorimeter has two vessels. figure \(\pageindex{1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat. Figure shows the construction of a calorimeter. click here👆to get an answer to your question ️ write brief answers. Explain The Construction Of Calorimeter Draw The Necessary Figure.